Exposure of Cominarty and Moderna vaccines to room temperature for more than 3 minutes is irreversible as the vaccine is considered thawed. It can’t be re-frozen.
In January 2021, the Cold Health Commission “Covid-19” of the « Association Française du Froid » (AFF) published several understanding notes, information and good practices of the cold chain around COVID-19 vaccines.
1. Pfizer BioNtech and Moderna Therapeutics COVID-19 vaccines packaging and storage
The Pfizer-BioNtech and Moderna Therapeutics COVID-19 vaccines have different packaging and special storage precautions.
COLD & CO presents the main characteristics that cannot be substituted for the product characteristic summaries, specific to each vaccine.
Vaccine’s name :
COMINARTY
VACCIN COVID-19 MODERNA
Laboratory :
Pfizer-BioNtech
Moderna Therapeutics
Packaging :
Doses / Vial:
Vials/ Box:
5 doses
195
10 doses
10
Storage at low temperature :
6 months between -90°C and -60°C
7 months between -25°C and -15°C
Preservation
after thawing :
5 days between 2°C and 8°C
30 days between 2°C and 8°C
Preservation after thawing and … :
…. after dilution: 6 hours between 2°C and 30°C.
…after using the first dose: 6 hours between 2°C and 25°C.
The thawing process has begun… :
… from -60°C.
… from -15°C.
2. Packaging of vaccines in a medical cooler or isothermal container
Pre-conditioning is required
A dose of vaccine left at room temperature exceeds the 8°C limit in less than 3 minutes. To avoid variations in the temperature of low and very low-temperature vaccines, ALL vaccine packaging elements should be preconditioned beforehand:
- Insulated packages or coolers must be previously cooled to prevent thermal shock on departure. To stabilize the temperature of your thermal container, you can load it entirely in dry ice -78.5°C. In the case of the Moderna vaccine, you can also pre-store your isothermal container at a temperature -25°C.
- in the case of use of previously frozen eutectics -20°C, care must be taken to ensure that the freezing time and temperature is sufficient to freeze these eutectics at heart. Our recommendation is to freeze them at least 48 hours at -30°C or 24 hours at -40°C. The proper freezing of the cold accumulators can be simply checked by shaking the gels or plates. If you perceive a liquid or semi-liquid state, the eutectic solution is not fully charged.
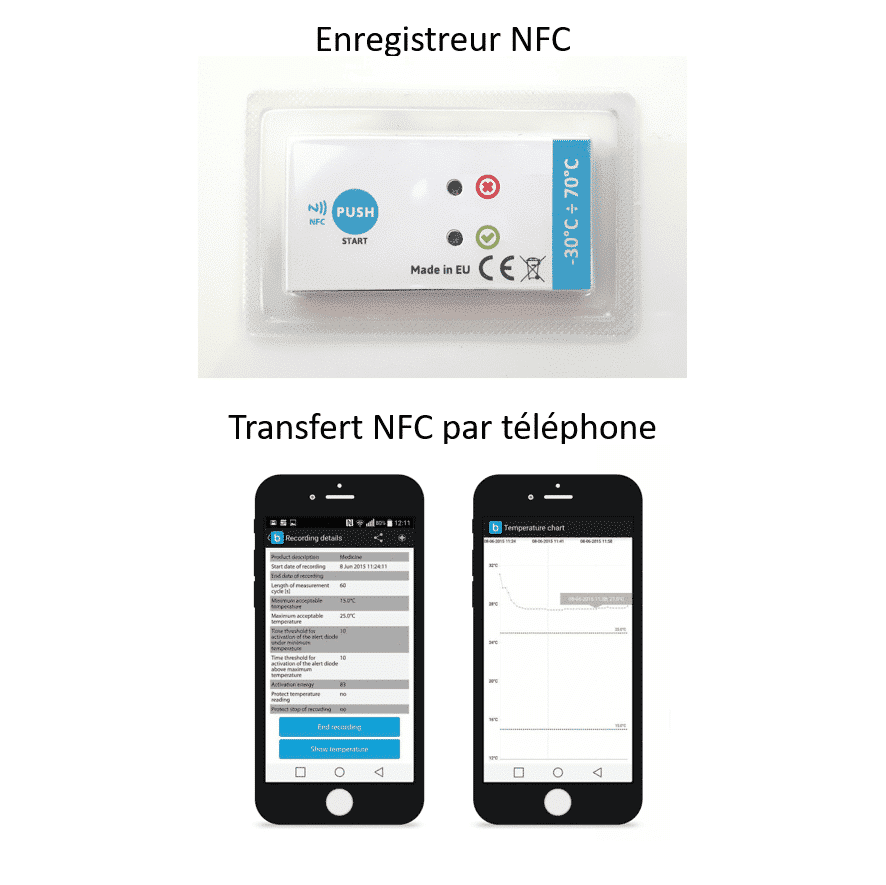
- temperature loggers need some time to stabilize their own temperature. The ideal is to position them already in the insulated container during the pre-cooling process so that it starts with the right temperature.
Loading your isothermal containers to accommodate low and very low temperature vaccines
Vaccine handling at room temperature should be done in less than 3 minutes. To avoid temperature excursions:
- Work at the lowest possible room temperature. We recommend a temperature of 5°C. If this is not possible, limit to a maximum ambient temperature of 25°C;
- Work in a draught-free environment. Air movements facilitate temperature transfers, avoid drafts and ventilations;
- Handle with gloves to avoid cold burns. This will also limit temperature transfers;
- Prepare all your items at your fingertips to minimize distances. Move transport boxes closer to storage areas. Movements should be measured in centimetres, not meters. The transit time must always be less than 3min;
- Remember to switch on the temperature recorders, if they do not have a remote system to start their recording, before inserting the vaccines;
- Dry ice should cover all 6 sides of thermosensitive vaccines.
- Have dry ice at the bottom of the isothermal container before positioning the vaccines;
- The dry ice sublimates in priority on the upper face. Also, we recommend keeping a higher dose of dry ice to be positioned on top of vaccines.
3. Writing instructions, training in low and very low temperature handling
To secure your vaccine loads in isothermal containers, explain what to do and why to do it by sequencing your packaging steps and formalizing them under loading instructions.
To write your internal vaccine handling instructions in your isothermal containers, we recommend performing loading simulations using placebo water bottles. Pack and load your placebo vials according to a series of predefined steps. Time and record the temperature of your placebo vials. Read your temperature curves to analyze your critical steps.